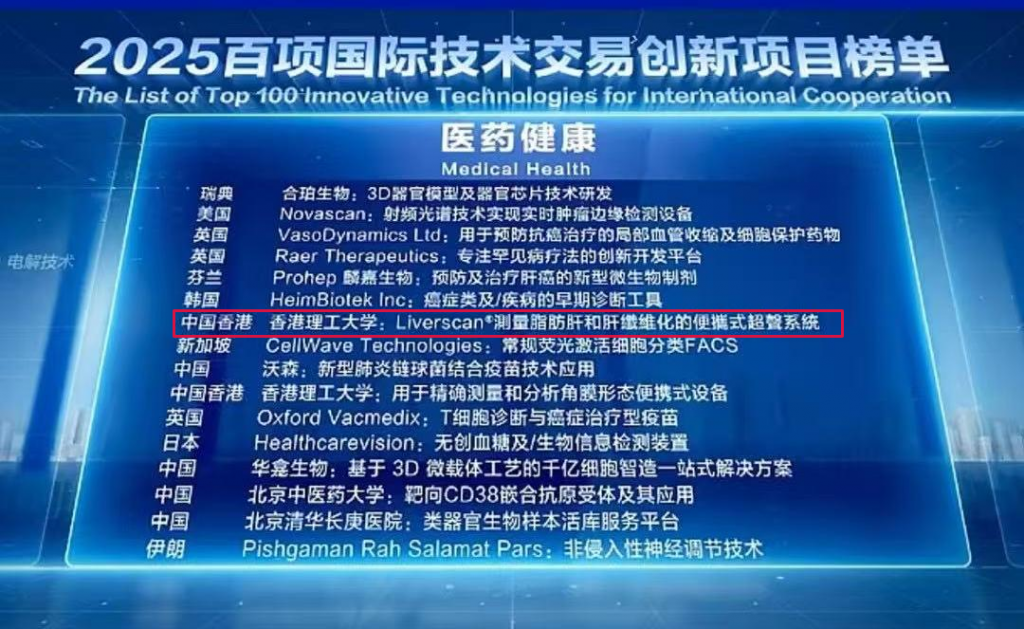
Good news! Liverscan® from Eieling Technology was successfully selected into the “List of 100 International Technology Transaction Innovation Projects”
On March 27, at the Zhongguancun International Technology Exchange Conference held at the 2025 Zhongguancun Forum Annual Meeting in Beijing, […]