
From March 26 to 30, 2025, the world-renowned 34th Annual Meeting of the Asia-Pacific Association for the Study of the Liver (APASL 2025) was held in the Beijing National Convention Center. As the largest and highest-level academic event in the field of liver disease and related fields in the Asia-Pacific region in 2025, this conference is themed “Multidisciplinary Collaboration For Elimination & Cure”. World-class medical experts from all over the world will discuss the latest developments in hepatology on this platform to promote knowledge exchange, technological innovation, and cooperation in the field of liver disease. At this exhibition, Eieling Technology brought the Liverscan® Portable Liver Elastography Ultrasound Diagnostic System to the exhibition, which received wide praise and high evaluation from customers at home and abroad.




Crowds of people gather to discuss development
With its outstanding innovation capabilities and leading technology, Eieling comprehensively and deeply demonstrated its advanced solutions in the field of liver disease detection. Liver disease experts and customers from all over the world gathered here to discuss new products and technologies in the field of liver disease. There was an endless stream of customers consulting and negotiating on-site. The Eieling team shared the latest technology trends with customers, discussed future industry trends, answered questions, and provided professional guidance and consultation.



In order to gain a deeper understanding of the superior performance of Liverscan®, Eieling Technology invited on-site experts and customers to experience and practice it in person. Through practical operations and interactive demonstrations, participants intuitively felt the outstanding performance of Liverscan® in terms of accuracy, convenience, and innovation. On-site customers recognized the detection efficiency of Liverscan® and believed that it could show extremely high use value in various scenarios, such as clinical and community. The fast, accurate,e and non-invasive detection characteristics not only improve the diagnostic efficiency, but also provide patients with a more convenient health management solution. Whether in hospitals, clinics or community health screening, Liverscan® has excellent adaptability and practicality, and has become an important tool to promote liver health management.



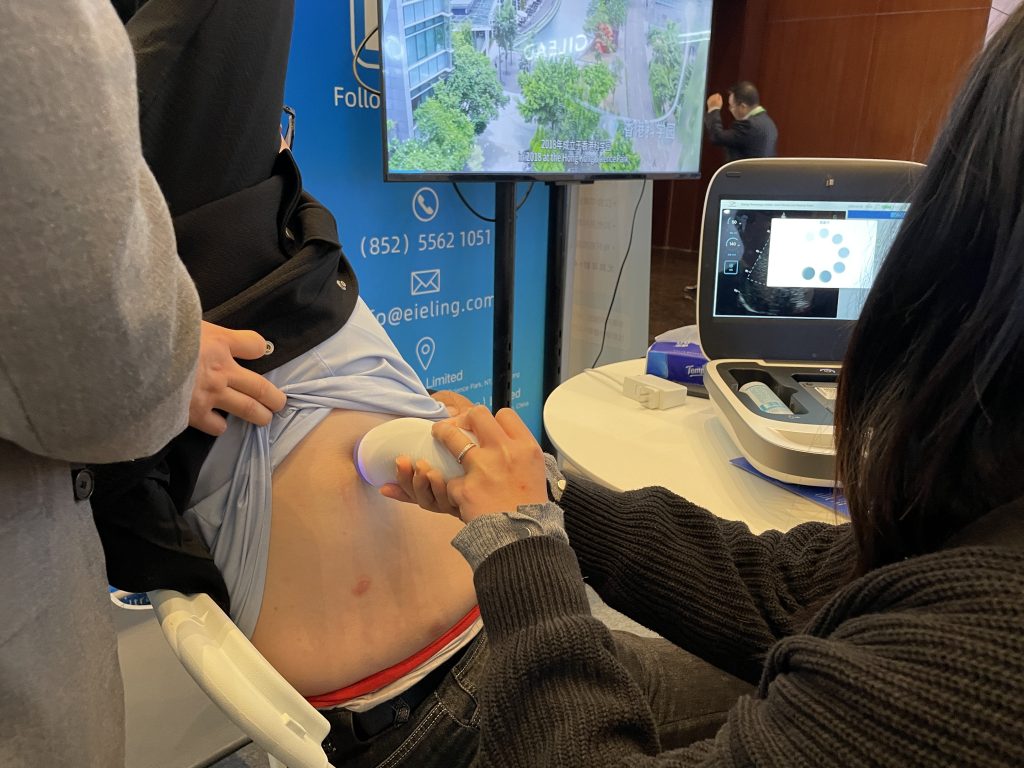
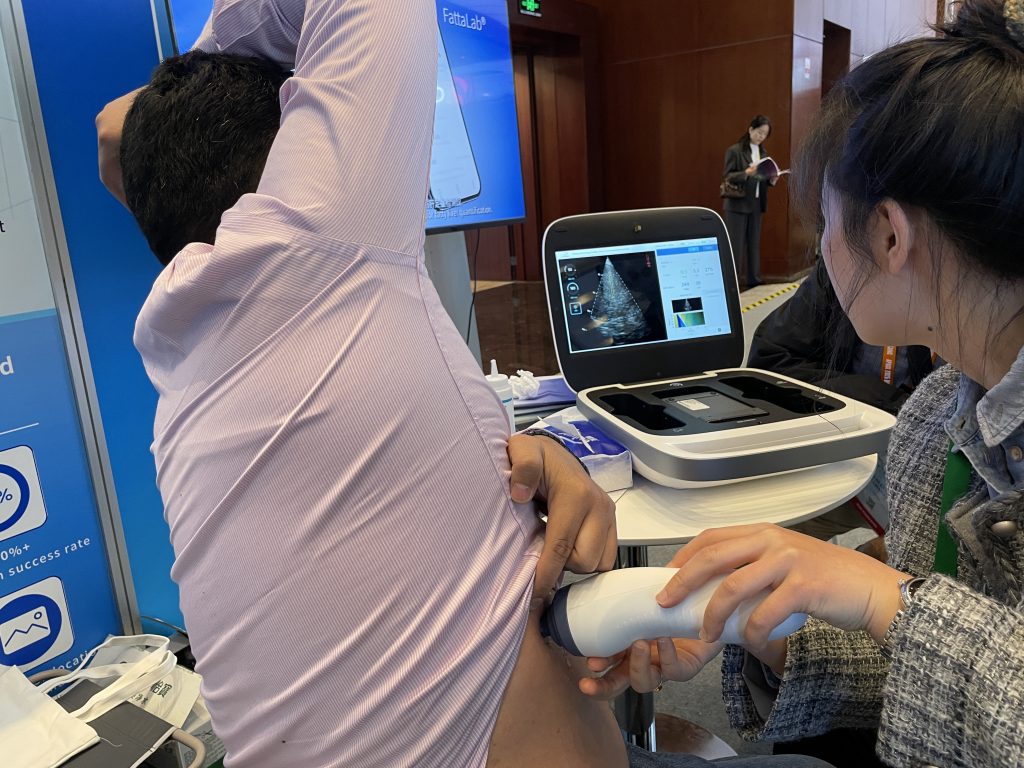

Accurate detection, broad prospects
Liverscan® has brought revolutionary breakthroughs in the field of liver diagnosis and treatment with its outstanding innovative design and performance. The probe uses wireless connection technology and can be flexibly adapted to various medical scenarios, greatly improving the mobility and ease of operation of the device. This design has made liver ultrasound examination no longer limited to traditional clinics, significantly expanding the scope of medical services.


In terms of technology, the Liverscan® portable elastic imaging ultrasound diagnostic system is equipped with a global exclusive patent, The Liverscan probe is equipped with a patented B-type ultrasound real-time image guide to measure the hardness of liver tissue .It provides liver tissue hardness and liver fat measurement functions through real-time image-assisted positioning. It can complete efficient testing in just one minute, which can help with early screening and accurate diagnosis of liver diseases.
Continuous innovation, benefiting the people
At present, Liverscan®, developed by Eieling Technology, has been successfully applied in practice, significantly improving the efficiency and convenience of medical diagnosis. With its technological innovation and excellent performance,the Liverscan® wireless dual-mode probe is reshaping the liver diagnosis and treatment model and has made important contributions to improving the quality of medical services and optimizing the allocation of medical resources.
The appearance at the Beijing Asian Liver not only demonstrated the strength of Eieling Technology, but also provided an important platform for it to expand the market and strengthen cooperation with all parties. Currently, Liverscan® has passed the China NMPA Class III medical device certification and the US FDA 510 (k), and the fatty liver home self-test “FattaLab®” will be officially released in May, further enhancing the market potential. Looking to the future, Yiling Technology will continue to increase R&D investment, promote the continuous innovation of liver disease diagnosis and treatment technology, and provide strong support for enhancing the level of liver disease prevention and treatment in my country.
